Workshop on Opioid availability in Rajasthan
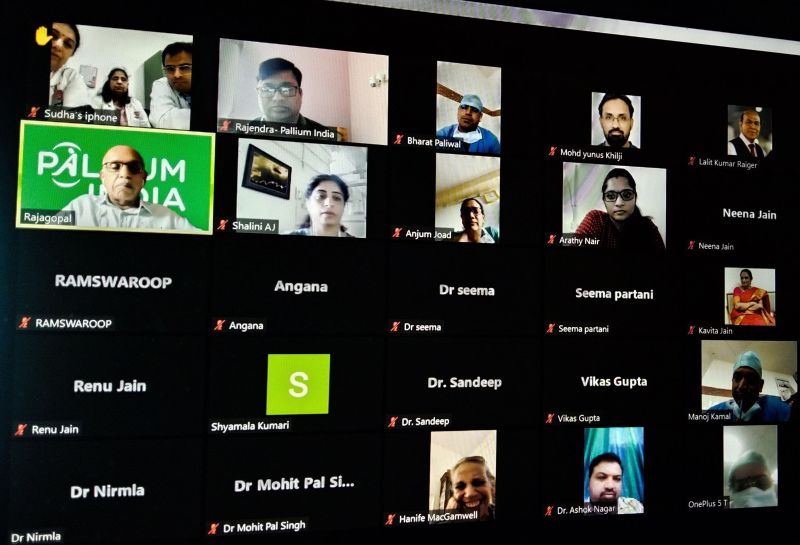
In co-ordination with Dr Anjum Khan Joad of BMCHRC (Bhagwan Mahaveer Cancer Hospital & Research Centre) Jaipur, Pallium India organised a Workshop on Opioid availability in Rajasthan on 4th August, 2022.
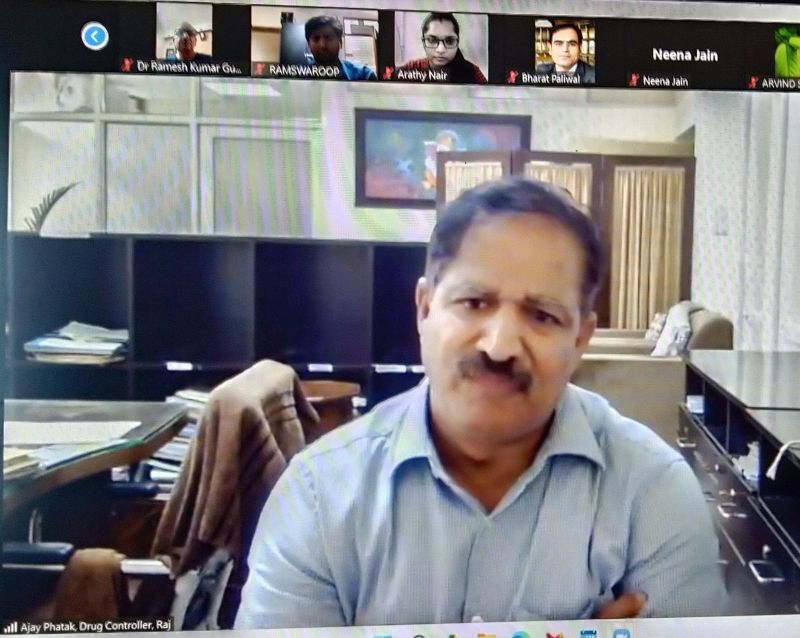
35 participants were present in the event including Mr Ajay Pathak, State Drug Controller Rajasthan, and Dr Nirmala, In-charge Officer NPPC, NHM Rajasthan, and twenty doctors from government & private sectors, Rajasthan.
Dr Anjum Joad welcomed all to the event. Ms Shalini AJ, Head-State Facilitation, Pallium India, introduced the programme. Dr MR Rajagopal, Chairman, Pallium India and Mr Ajay Pathak explained 2014 NDPS Amendment and subsequent 2015 Rules in the state.
Dr Rajagopal clarified that the licensing process through Excise department is no more valid. He added that the State Drug Controller is the authority who can issue Recognized Medical Institution (RMI) license to applicants for stocking Essential Narcotic Drugs (6 drugs of which Morphine, methadone and Fentanyl are available in India)
The Drug Controller opined that there is lack of awareness on the 2014 NDPS Amendment and subsequent 2015 Rules in the state.
Dr Rajagopal clarified that government hospitals are deemed RMI and need not apply for certification with the SDC. He added that, though the government hospitals need not apply for the certification, they are bound to keep all the documentation up to date as prescribed by the NDPS 2014 amendment.
SDC said that the organisation/ hospital can apply through https://drugscontrol.org site or app.
Dr Anjum and Ms Hanife MacGamwell also appreciated the Pallium India’s support in starting palliative care OPD and processing the RMI license.
Pallium India has shared PPT, other necessary documents and application forms for RMI status with the participants.